SUSTech chemists find new strategy for concise total synthesis of Vinigrol
Natural products and their derivatives have long been the main source of innovative drugs and an important tool for biomedical research. Nearly 60% of clinically applied drugs are directly or indirectly derived from natural products. However, the source of natural products is very limited and it is difficult to meet the needs of various research. Therefore, the research on the synthesis of active natural products is crucial for the in-depth development of Chinese medicine modernization.
Recently, Professor Li Chuangchuang from the Department of Chemistry at Southern University of Science and Technology (SUSTech) led his research group to a highly efficient asymmetric synthesis of Vinigrol. Their results were published in Journal of the American Chemical Society (JACS), in a paper titled “Asymmetric Total Synthesis of (−)-Vinigrol,” showing the shortest synthesis in the history of vinigrol synthesis.
Vinigrol is known as one of the most difficult molecules to synthesize, with more than 20 research groups around the world having published more than 30 papers since 1987 on its synthesis. It was not until 2008 that the full synthesis of the Vinigrol molecule was achieved.
Vinigrol has a unique tricyclic skeleton consisting of a fused ring system and an eight-membered bridge ring. Vinigrol also has eight consecutive stereogenic centers and three hydroxyl functional groups. Vinigrol presents a new challenge for synthetic chemists. The structure first appeared in natural products, and there is no mature method to synthesize it, thus making its synthesis challenging.
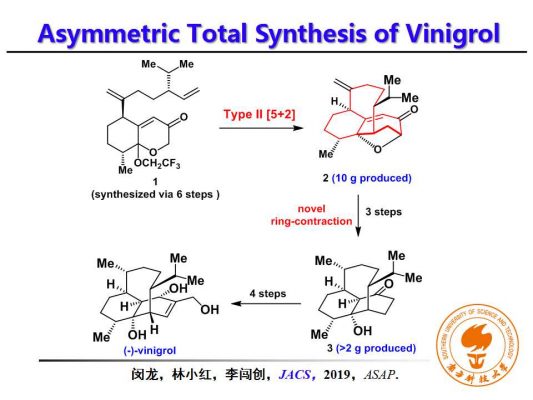
The research group developed a new asymmetric synthesis strategy by using readily available chiral building materials in their own reaction that offers a highly efficient and concise result without protective groupings. Type II [5+2] cycloaddition is the key strategy to synthesizing the highly rigid bridgehead double bonds efficiently. The asymmetric total synthesis of the Vinigrol molecule took place in 15 steps, an improvement on the previous effort of 20 steps.
It is worth mentioning that the reaction developed by Professor Li Chuangchuang’s research team is unique and provides a new strategy for the rapid construction of the bridge ring system. Efficient total synthesis of a plurality of complex active natural products has been accomplished using this reaction (see J. Am. Chem. Soc. 2018, 140, 5365; J. Am. Chem. Soc. 2019, 141, 2872).

Since joining SUSTech, Professor Li Chuangchuang has been devoted to the development of innovative synthetic methods and the study of total synthesis of complex active natural products. the research and development of original methodologies have been carried out for the total synthesis of complex active natural products, focusing on the basic scientific problems in organic synthetic chemistry.
With the synthesis of bioactive natural products as the center and drug R&D as the guidance, the research team has fully developed and applied new strategies and technologies of modern organic synthesis in order to construct a natural product bank quickly, efficiently and diversify, from which new drug molecules or lead compounds can be found.
Research Assistant Professor Dr. Min Long is the first author of the paper. Ph.D. student Lin Xiaohong also made important contributions to the paper. Professor Li Chuangchuang is the sole correspondent of this paper, and SUSTech is the only completion unit.
The research has received funding from the Natural Science Foundation of China, and the Shenzhen Science and Technology Innovation Committee.
Original article – https://doi.org/10.1021/jacs.9b08983